Checking Battery Condition
If the vehicle is not performing satisfactorily, and it is suspected to be battery related, test the battery set and determine battery condition.
An effective way to identify the cause of a poorly performing battery is to perform a hydrometer test. Using a hydrometer will identify a battery in a set with a lower than normal specific gravity. Once the particular cell or cells that are the problem are identified, the suspect battery can be removed and replaced.
Hydrometer
-
The hydrometer must be ordered separately from your John
Deere dealer.
The state-of-charge of a lead acid battery can be determined by the specific gravity of the electrolyte (its weight compared to water). The specific gravity can be measured directly with a hydrometer (A).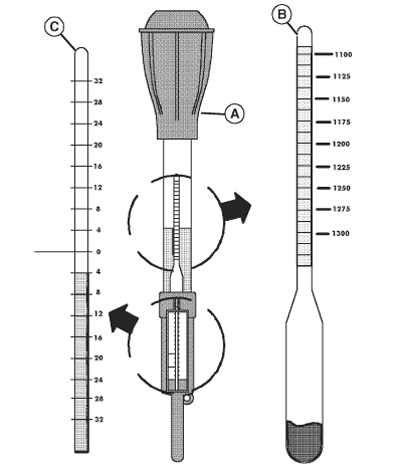
MXAL47827-UN-12APR13 -
A hydrometer is a bulb-type syringe which will extract electrolyte
from a battery cell. A glass float (B) in the hydrometer barrel is
calibrated to read in terms of specific gravity.
- The range of specific gravity used on these floats is 1.140 to 1.325.
- DO NOT assume a battery will not take a charge because you have been charging it for a while and the float will not rise. The battery may have been fully discharged and will require considerable charging before reaching the minimum specific gravity on the float.
- The lower the float sinks in the electrolyte, the lower its specific gravity.
- Hydrometer floats are calibrated to give a true reading at one fixed temperature only. The hydrometer is equipped with an internal thermometer (C) that will measure the temperature of the electrolyte and will include a conversion scale to correct the float reading.
- A correction factor must be applied for any specific gravity reading made when the electrolyte temperature is not 26.7°C (80°F). A temperature correction must be used because the electrolyte will expand and become less dense when heated. The float will sink lower in the less dense solution and give a lower specific gravity reading. The opposite occurs if the electrolyte is cooled. It will shrink in volume, becoming more dense. The float will rise higher and give a false high reading.
- A correction factor of 0.004 specific gravity (sometimes referred to as 4 “points of gravity”) is used for each 5.5° (10°F) change in temperature. Four “points of gravity” (0.004) are added to the indicated reading each 5.5°C (10°F) increment above 26.7°C (80°F) and four points are subtracted for each 5.5°C (10°F) below. This correction is important at temperature extremes because it can be a substantial value.
- Example 1: A partially discharged battery is installed in this vehicle at 6.7°C (20°F). A hydrometer reading of 1.250 would indicate that the battery is almost fully charged. However, when the correction factor is applied, the true value is 1.226 only 75% charged.
- Example 2: A battery exposed to the sun in hot weather can easily exceed 43°C (110°F). A hydrometer reading of 1.238 might indicate a low state of charge, or there is a problem with the electrical system if the battery is already in service. However, when the correction factor is applied, the true value is 1.250, 100% charged.
|
OUO1023,0000996-19-20130417 |